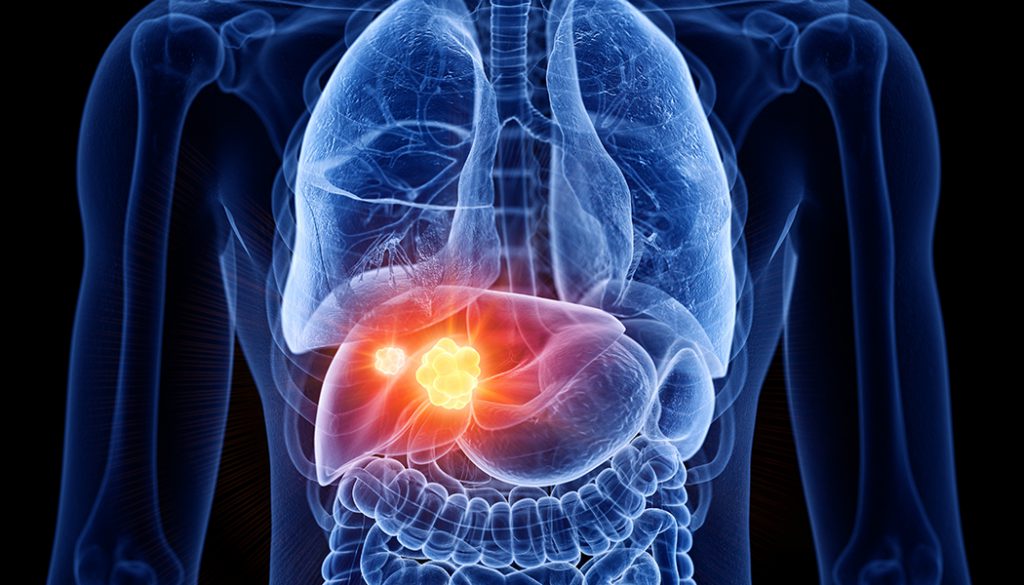
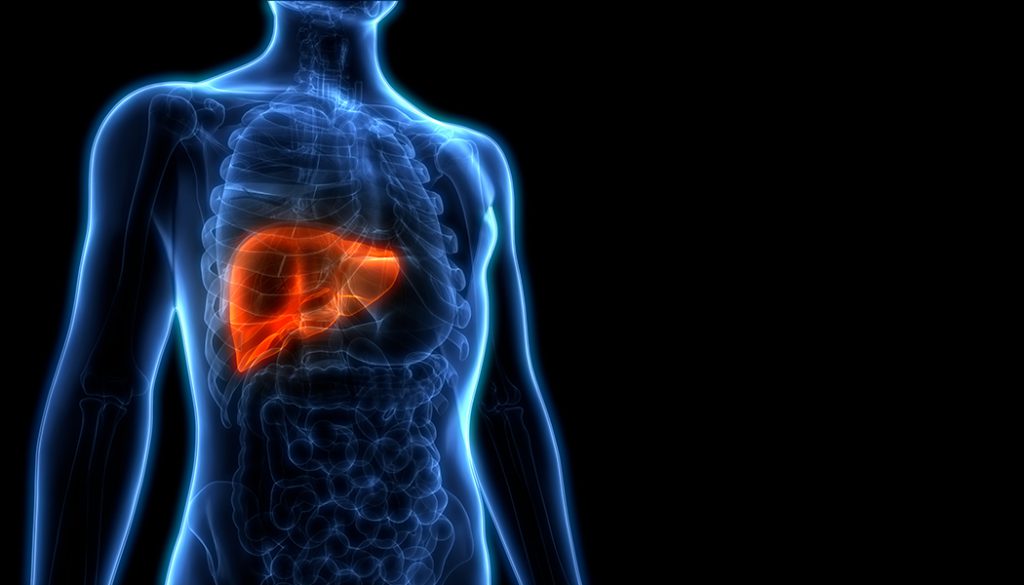
Liver Cancer
Liver cancer is the fifth most common cancer in men and the ninth most common cancer in women worldwide. The incidence rate of liver cancer is larger in developing countries. The percentage of Americans who get liver cancer rose for several decades, but is now declining.
Key Facts
- An estimated 41,210 new cases of liver cancer will be diagnosed in the U.S. in 2021, with 29,380 deaths expected to result from the diagnosis.
- For the 43% of people who are diagnosed with liver cancer at localized-stage, the five-year survival rate is 36%.
- For those people diagnosed with liver cancer at regional stages, the five-year survival rate drops to 13%.
- In the U.S., liver cancer incidence has more than tripled since 1980.
- Liver cancer is approximately three times as likely to occur in men than in women.
- The liver is a common place where cancer spreads. Colorectal, breast, esophageal, stomach, pancreatic, kidney, lung and melanoma skin cancers are the most common sources of cancer
- Approximately 70% of liver cancer cases in the US could potentially be prevented through elimination of risk factors, the most important include: excess body weight; type 2 diabetes; infection with hepatitis B virus (HBV) and/or hepatitis C (HCV), heavy alcohol consumption and tobacco smoking.
Source: American Cancer Society’s Cancer Facts & Figures 2023 and GLOBOCAN, 2020
Signs and Symptoms
A symptom is a change in the body that a person can see and/or feel. A sign is a change that the doctor sees during an examination or on a laboratory test result. If you have any of the symptoms below, it does not mean you have cancer but you should see your doctor or health care professional so that the cause can be found and treated, if needed.
- Weight loss (without trying)
- Loss of appetite
- Feeling very full after a small meal
- Nausea or vomiting
- An enlarged liver, felt as fullness under the ribs on the right side
- An enlarged spleen, felt as fullness under the ribs on the left side
- Pain in the abdomen (belly) or near the right shoulder blade
- Swelling or fluid build-up in the abdomen (belly)
- Itching
- Yellowing of the skin and eyes (jaundice)
Source: American Cancer Society 2023

Liver Cancer Awareness Month is recognized in October. To help accelerate cures please make a gift today.