Professor of Medicine, Division Director of Medical Oncology
Associate Director for Early Phase Clinical Trials at The James Comprehensive Cancer, The Ohio State University
Cancer-Types Supported
Click a cancer type below to learn more:

Research Projects
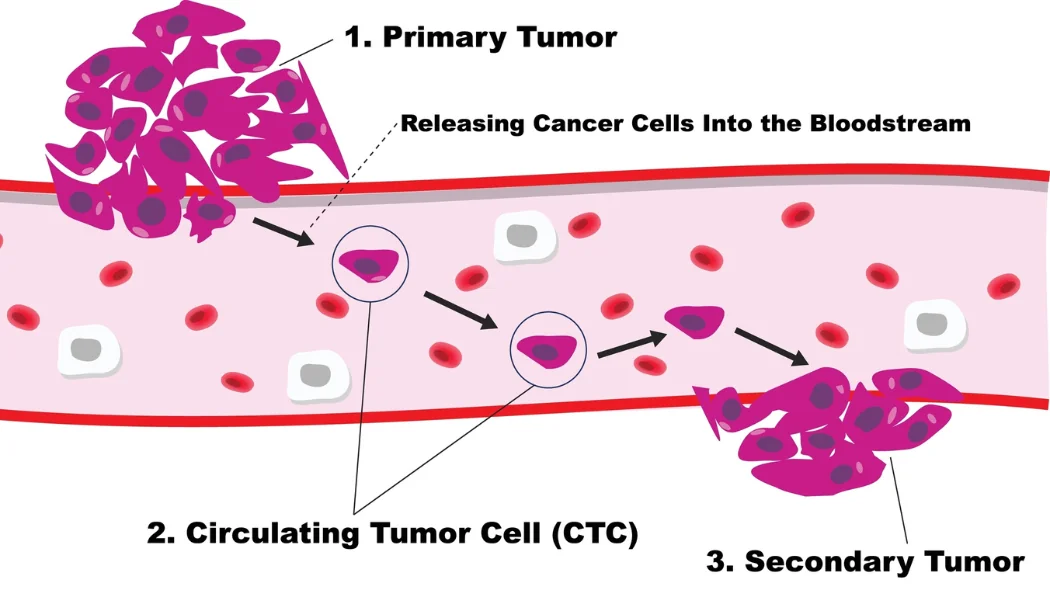
Small cell lung cancer (SCLC) is a particularly aggressive and deadly disease that accounts for about one in seven individuals with lung cancer. Although there are several treatment options, there are no reliable diagnostic tests, or “biomarkers”, that identify tumors that will respond to specific treatments. In addition, current treatment options are not curative, and even when treatment is initially effective in controlling the disease, resistance to treatment often develops rapidly.
Dr. Christian Rolfo’s team has developed laboratory models from SCLC circulating tumor cells (CTCs) derived from patients, allowing the generation of models that replicate the behavior of the human tumor, and facilitate identification of specific molecular changes that contribute to treatment resistance. The scientists plan to standardize and validate this novel strategy of evaluating CTCs derived from patients in the models and transform it into a reality.
IMPACT
This new approach may individualize treatment selection and _develop a new approach for prolonging survival of patients with SCLC.
Background
Christian Rolfo, M.D., Ph.D. is the Associate Director of Clinical Research at the Center for Thoracic Oncology- The Tisch Cancer Institute. Mount Sinai Health System & Icahn School of Medicine at Mount Sinai.
He is the president of the International Society of Liquid Biopsy and actively works on liquid biopsy biomarkers, particularly in CTCs, ctDNA, and extracellular vesicles (EVs) in lung cancer. He significantly contributed to the clinical development of several drugs that are widely used in medical oncology, such as Erlotinib in first-line NSCLC epidermal growth factor receptor (EGFR) mututated patients; Entrectinib, approved for ROS1 NSCLC; and other treatments such as MET, PARP, CD70, ICI, and RET inhibitors. He participated in several early clinical trials, including first-in-human studies, first dosing, as principal investigator (PI), serving as the global PI for some of these studies.

The National Foundation for Cancer Research wishes to thank the Sorenson Legacy Foundation for its generous support to expand on this critical research initiative.
Accelerate innovative research like this and help save cancer patient lives.
Research Focus Areas
Select a Focus Area Below to learn more and see others working in these area.